
Adenosine is molecule of multiple carbon rings, as represented by the right side of the molecule below. Adenosine Triphosphate (ATP)Ī molecule that nearly every organism uses is adenosine triphosphate or ATP. The wide variety of differently shaped carbon molecules in the biological world produces unique interactions. This can create molecule that are flexible, and vary in shape. Carbon, when it forms double bonds with other carbon atoms, can rotate around the bond. All of the types of molecules described below contain carbon, with a wide variety of other atoms covalently bonded to the carbon. All organic molecules contain carbon, and the ability to manipulate carbon bonds was probably a very early development in the evolution of life. Carbon has a unique ability to form 4 covalent bonds, which can lead to long chains of molecules. Examples of Molecule Carbon-Based MoleculesĬarbon is probably the most important element for all living organisms. This is why our bodies have millions of enzymes, bacteria, and fungi that function together to break the many covalent bonds present in our food and release the energy. It also means that the covalent bonds in food must be broken apart to gain energy. Organisms can use this to their advantage by storing energy in chemical bonds. Not only are covalent bond stronger that ionic bonds, but they store more energy. Sharing an electron is known as a covalent bond and is very important in biology. Molecules can form single bond, double bonds, triple bonds, and even more, depending on how many electrons they are sharing.
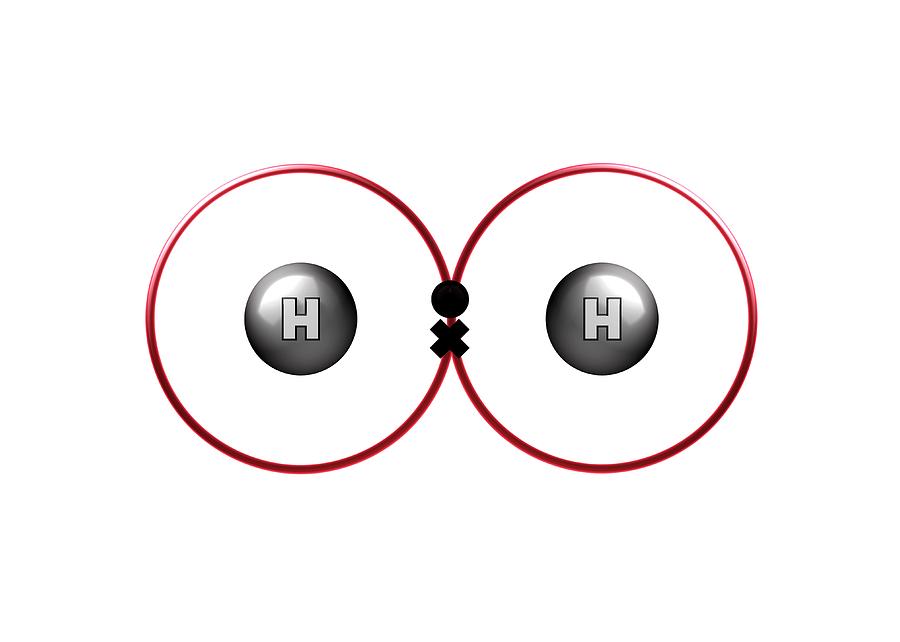
This electron activity ties the two atoms together. When two atoms share an electron, or multiple electrons, a strong bond is formed between them as the electron passes from one nucleus to the other and back. These bonds to not make a molecule, and the ions can be easily separated. These opposite electrical effects attract each other and form ionic bonds. One will be positive and one will be negative. These atoms both change in electrical charge and become ions. Sometimes, one atom will give away electrons to another atom. These shells prefer to have specific numbers of electons, depending on the shell. The electrons that orbit the nucleus exist in various clouds, or valence shells. The nucleus consists of protons and neutrons, of different numbers in different elements. Each atom carries a certain number of electrons that orbit around the nucleus. A molecule is two or more atoms bonded together to form a single chemical entity.


 0 kommentar(er)
0 kommentar(er)
